In an era of stringent regulations, ensuring your ECM and IDP systems are compliant and audit-ready is critical for organizational success.

Understanding the Importance of Compliance in ECM and IDP Systems
Enterprise Content Management (ECM) and Intelligent Document Processing (IDP) systems are vital for managing a plethora of critical documents such as contracts, invoices, HR records, and regulatory forms. Ensuring these systems are compliant is not just about adhering to regulations; it’s about safeguarding the integrity and security of sensitive data.
Compliance in ECM and IDP systems ensures that organizations can avoid legal penalties, protect their reputation, and build trust with stakeholders. With the increasing complexity of regulatory frameworks, maintaining compliance has become a strategic priority for organizations of all sizes.
Key Regulatory Requirements Impacting ECM and IDP Systems
Several key regulations impact the management and processing of documents within ECM and IDP systems. The General Data Protection Regulation (GDPR) focuses on protecting the personal data and privacy of individuals within the European Union. HIPAA (Health Insurance Portability and Accountability Act) sets the standard for protecting sensitive patient data in the healthcare industry.
Other regulations like the Sarbanes-Oxley Act (SOX) enforce strict requirements for financial reporting and data integrity. Organizations must ensure their ECM and IDP systems are capable of meeting these diverse regulatory requirements to avoid costly fines and reputational damage.
Best Practices for Monitoring Compliance
To ensure compliance, organizations should implement a comprehensive monitoring strategy. This includes regular audits, real-time alerts for non-compliance, and detailed reporting capabilities. Establishing clear policies and procedures for document management and data protection is essential.
Training employees on compliance best practices and maintaining up-to-date documentation can also help in ensuring adherence to regulations. Additionally, leveraging automated compliance tools for ECM and IDP can significantly reduce the risk of human error and enhance the efficiency of compliance monitoring.
Technological Tools to Support Compliance Monitoring
Several tools can aid in monitoring compliance within ECM and IDP systems. This means understanding, tracking, and compiling user information in a manageable system.
Reveille offers comprehensive compliance metrics for ECM, IDP, and RPA based on real usage. It extends beyond Reveille’s specialized monitoring for these systems, consolidating precise compliance data in a single location.
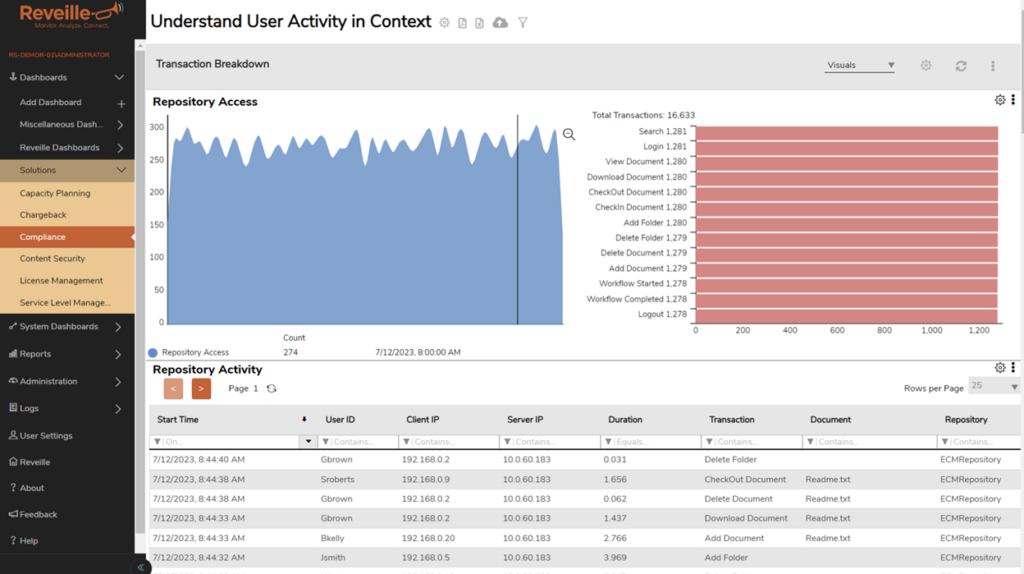
This includes automatically capturing key metrics such as transaction counts, types, information, client details, transaction durations, and much more. Compliance rules can be established within Reveille and notifications can be sent when thresholds are crossed that have a compliance impact.
Reveille’s value becomes evident with all the data organized in one location:
- Faster audit preparation.
- Reduced risk of fines and data breaches.
- Improved operational efficiency (because compliance drives process discipline).
- Peace of mind for CIOs, CCOs, and IT admins alike.
Automated workflows and document classification tools can also ensure that documents are processed and stored in accordance with regulatory requirements. Integrating these tools with existing ECM and IDP systems can streamline compliance efforts and enhance overall data governance.
Preparing for Audits: What You Need to Know
Preparation is key to a successful audit. Organizations should conduct regular internal audits to identify and address compliance gaps with the appropriate measures and tools. Maintaining accurate and accessible records of all compliance-related activities is crucial.
Ensure that your ECM and IDP systems can generate comprehensive audit trails and reports. This not only facilitates the audit process but also demonstrates your organization’s commitment to maintaining high compliance standards. Engaging with external auditors for periodic reviews can provide an additional layer of assurance and help in staying ahead of regulatory changes.




